Nanordica Medical is an Estonian MedTech company pioneering advanced antibacterial nanotechnology for the prevention and treatment of topical bacterial infections. The company has filed several international patents and developed its first product — an advanced antibacterial wound dressing.
In a randomized clinical trial conducted at Estonia’s largest hospital, Nanordica Medical’s wound dressing demonstrated superior efficacy, successfully healing chronic wounds that could not be cured with other methods. The study also showed that the dressing causes fewer adverse effects compared to the current leading antibacterial alternatives.
Through its innovation and scientific rigor, Nanordica Medical is redefining how bacterial infections are treated, setting a new standard for wound care technology worldwide.
Interview with Anna-Liisa Kubo, Co-founder & Chief Innovation Officer at Nanordica Medical.
A brief description of the company and its activities.
Anna-Liisa Kubo: Nanordica Medical was established by scientists and medical doctors with the purpose of taking innovations from lab to improve medical practice. Nanordica is a spin-off of the National Institute of Chemical Physics and Biophysics (Estonia) developing technologies for medical devices in wound management comprising advanced materials, copper and silver in nanoform. Nanordica`s first product Advanced Antibacterial Wound Dressing is designed to treat wounds at risk of infection, containing natural silk nanofibers blended with copper and silver collectively known as Premotiv® nanotechnology. Nanordica Medical antibacterial technology is with high safety and efficacy. The dressing has several layers of innovation and the notably high antibacterial effect is within the wound contact layer having thickness comparable to a sand grain. This makes the technology especially innovative because of low constituent of ingredients we achieve bacteria killing properties but sustain safety. Nanordica`s dressing has been tested in clinical trials in Estonia and is being tested in an ongoing trial in Spain on patients with diabetic foot ulcers (DFUs) – the most challenging wounds on human patients. In this study, we succeeded to demonstrate excellent healing of wounds compared to the standard of care– the traditional antibacterial wound dressing. This drives Nanordica Medical aspirations in advanced wound care with the mission to help hundreds of millions of patients.

What are the main areas of activity of the company?
A.L.K: Nanordica Medical has all the main competence to develop innovative wound care products, such as competence for toxicology, microbiology, cell biology, chemistry, clinical research, research and development, business development and regulations. This gives us good grounds to understand the needs of the products from the client’s perspective for designing products to meet the unmet clinical needs. In that way we bring to the clinical specialists’ new solutions and help to make their work better.

What’s the news about new products/services?
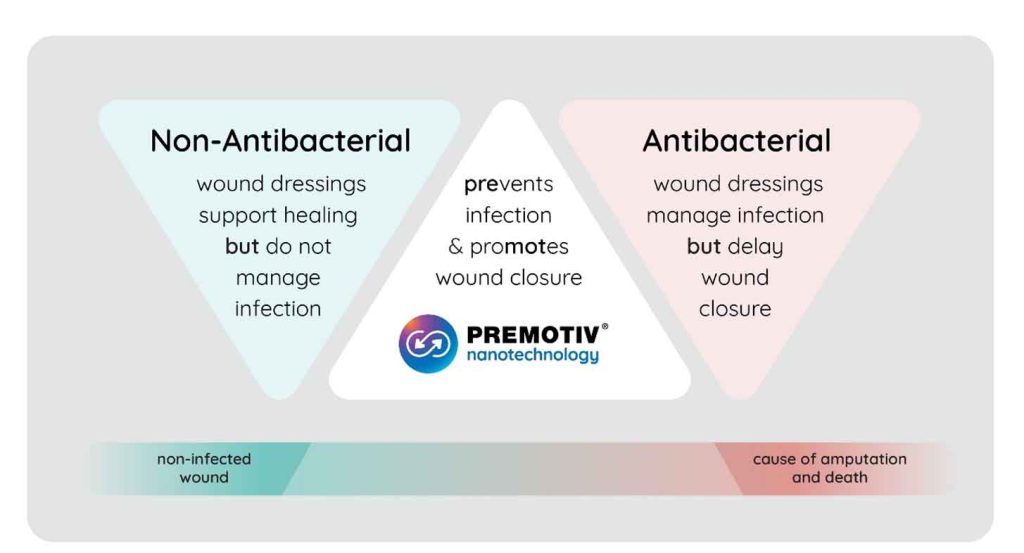
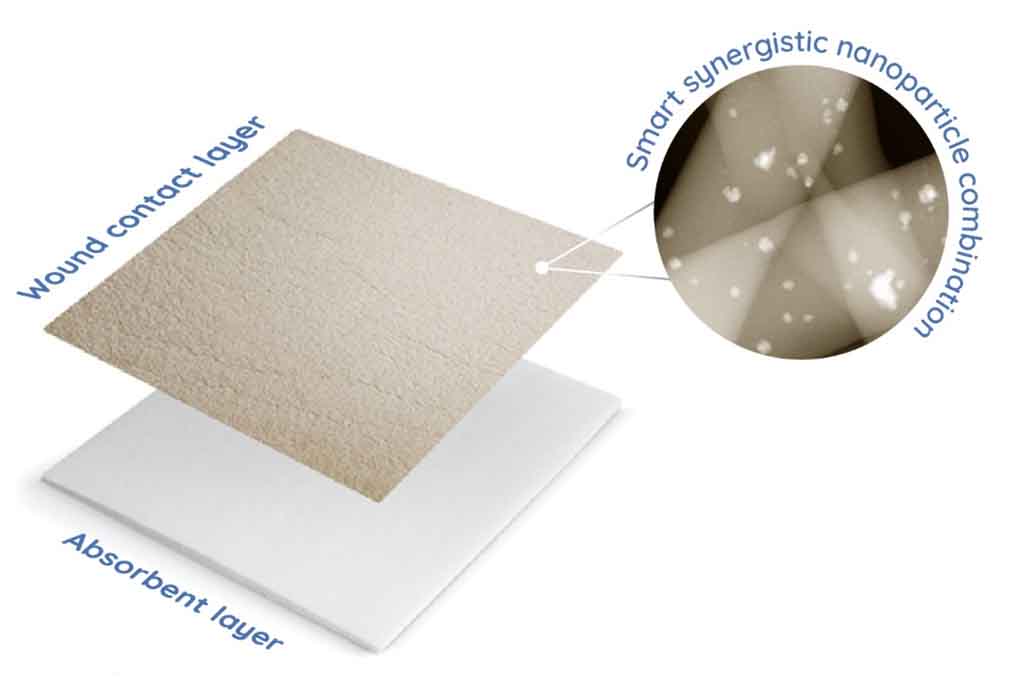
A.L.K: With our technology we would like to change how we see wound care today. The company started with research about the environmental aspects of silver nanoparticles and found that silver is the most used element in wound care. Although very efficient antibacterial, it is also a slightly toxic element to the cells and to the environment. In Premotiv® technology, we have decreased toxicity and increased efficacy by significantly reducing silver concentration and using it smartly/synergistically to enhance copper antibacterial properties reaching the point where we have the most innovative silver technology in the world. In this way, we designed a smart combination of nanoparticles by combining silk nanofibers with silver and copper and achieved high efficacy with high safety. We call this technology Premotiv® because it is designed to prevent infection and promote wound healing (Figure 3). Our first product is tested on diabetic foot ulcer patients, but we are already widening the product portfolio to other wounds.
What are the ranges of products/services?
A.L.K: Nanordica Medical develops first-in-class wound care products with new and patented smart innovative nanotechnology. Our primary focus in wound care is diabetic foot ulcers. However, we are continuously innovating and expanding our solutions to address a wide range of wound types, like venous leg ulcers, pressure ulcers, burn wounds and traumatic wounds. We even have a veterinary line of products to cure wounds from pets like cats, dogs and horses.
What is the state of the market where you are currently active?
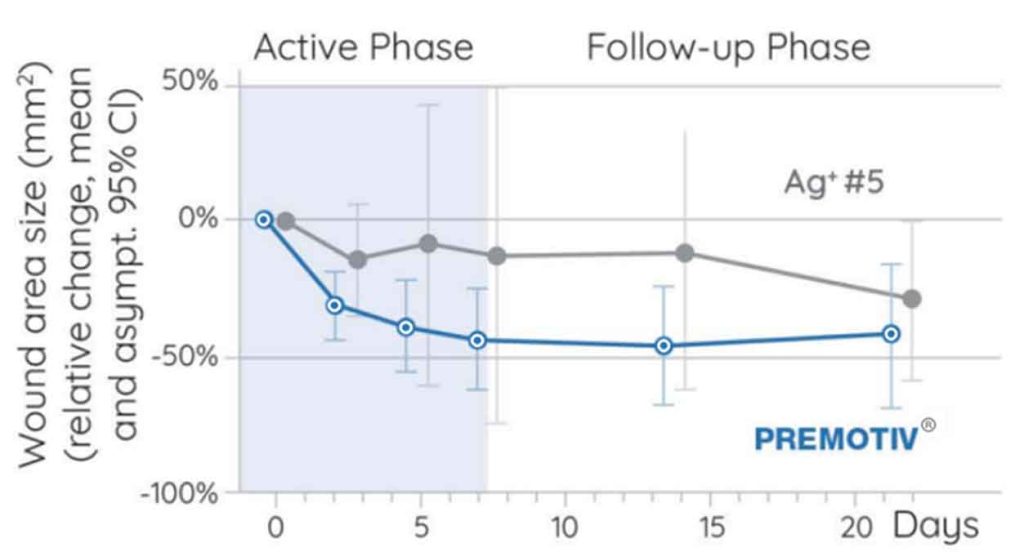
A.L.K: Nanordica Medical is in the chronic wound care market. The wound becomes chronic when healing has not taken place for 4 weeks. We tested our dressing on diabetic foot ulcer patients at the North Estonia Hospital showing a good tendency for faster closure after one week of treatment and three dressing changes (manuscript under preparation). Later it was stated that these are the most difficult wounds where we showed the difference. This study paved the way for a larger study in Spain where higher number of patients are recruited to stem the difference. This study could lead us even to change the way how we treat wounds by becoming a part of the standard of care.
What can you tell us about market trends?
A.L.K: The products are becoming smarter and more efficient leading us to more knowledgeable end-users. Market is moving towards evidence-based medicine. For example, Nanordica Medical is testing the novel concept in one of the largest study ever done on diabetic foot ulcers. The environmentally-friendly materials and production is also gaining relevance giving the advantage to the materials that have more sustainable product life cycle. Gladly, we are one of the leading companies in technology efficacy supported by high safety besides, in our production we are following the principles of eco-friendly manufacturing.
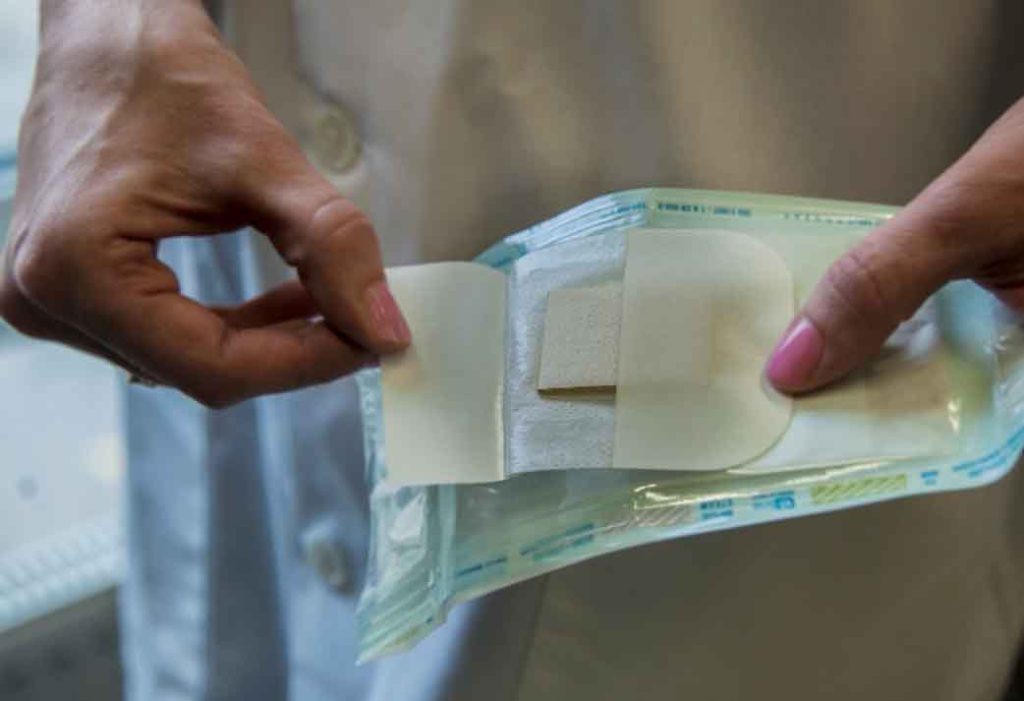
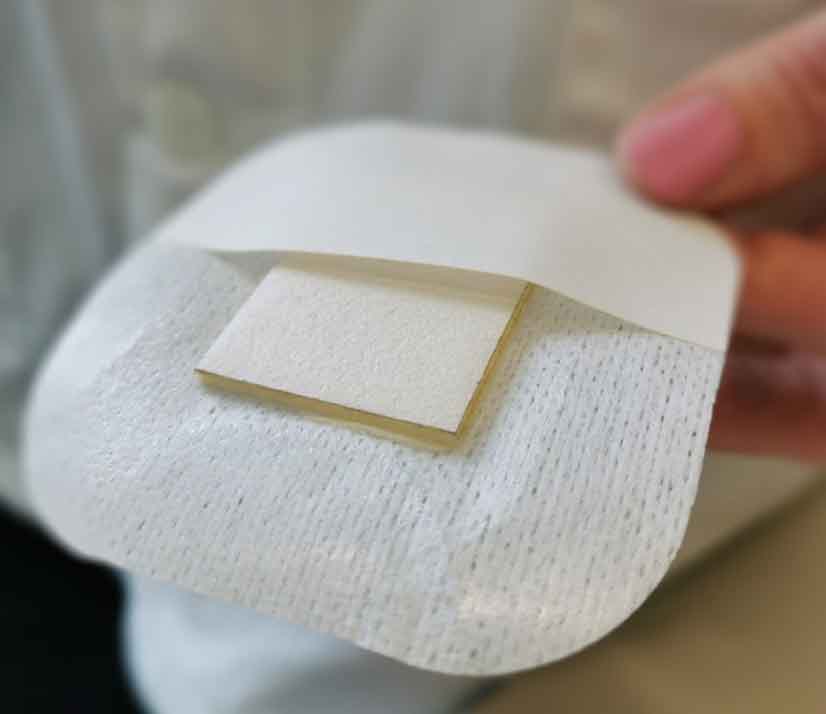
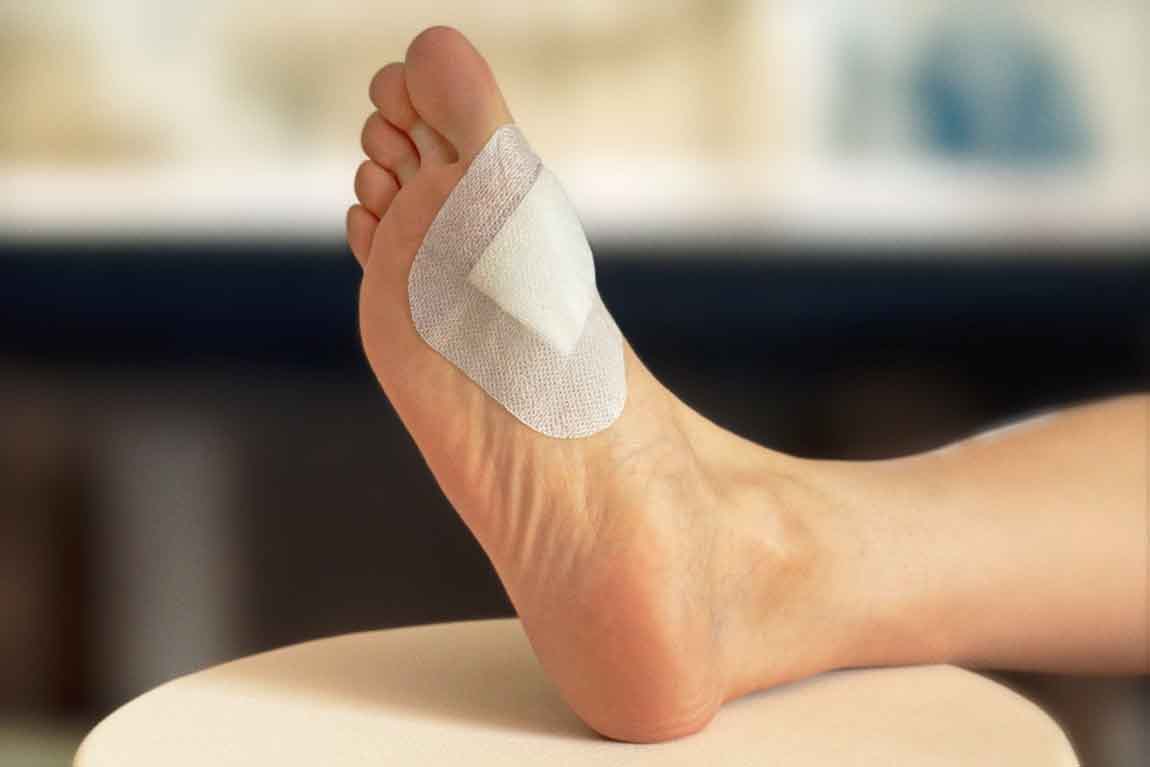
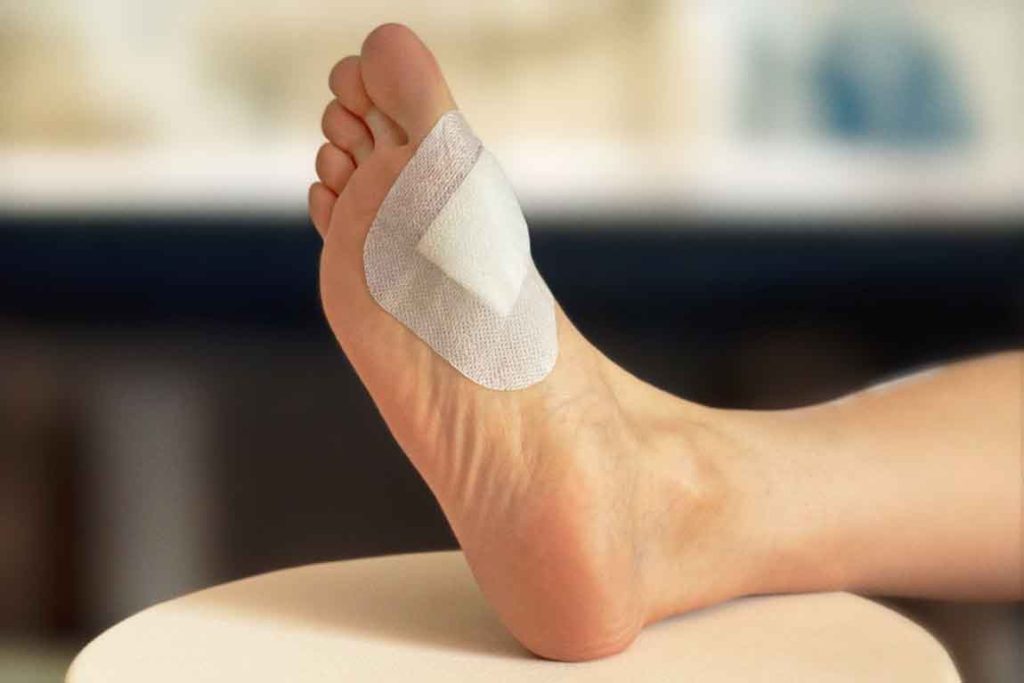
Figure 6. Nanordica Advanced Antibacterial Wound Dressing is a sterile single use wound dressing that is manufactured following environmentally friendly principles. It looks like a simple dressing but contains innovative nanotechnology.
What are the most innovative products/services marketed?
A.L.K: Our patented nanotechnology Premotiv® is designed to prevent infection and promote wound closure. The current product Nanordica Advanced Antibacterial Wound Dressing has several layers of innovation. Nanordica Medical Premotiv® nanotechnology is in the wound contact layer where copper and silver are embedded in silk nanofibers. This product is novel because silver and copper have been used in wound dressings previously separately but not in combination. Indeed, in such a way we achieve high efficacy with high safety. Thus, our product is very special because while efficiently killing bacteria – it is several times safer to human cells than traditional antibacterial wound dressing.
What estimations do you have for the second half of 2025?
A.L.K: Our main challenge currently is to obtain market approvals. By the end of 2025 we hope to gain permission to sell the products. Our target markets are Spain, Germany and Saudi-Arabia, the Baltic States and Finland.