The follow-up article to ‘UNDERSTANDING BRAKE WEAR PARTICLE EMISSIONS AND EURO VII STANDARDS’ focuses on the further development of ferritic nitrocarburizing processes and their effectiveness in reducing brake wear particle emissions. The discussion extends to the integration challenges of new technologies in mass production, the potential for automation, and the future direction to mitigate brake wear particle emissions.
Exploration of ferritic nitrocarburizing and benefits in reducing brake wear particle formation
With the introduction of energy recovery systems and the use of engine braking by intelligent automatic transmissions, there is now increased corrosion of brake discs. Common brake discs are made of cast iron that simply rusts. If the brake is not in use, the rust coating is no longer abraded by the brake pads and the corrosion attack goes deeper, resulting in significantly higher brake disc wear, especially on the rear brakes [1].
Considering the new requirements of electromobility where mechanical braking is reduced to a minimum, corrosion attacks will increase. This results not only in visual defects but also in reduced performance of the entire brake system and a shorter service life [2].
Thus, owners of vehicles increasingly observe that during periodical inspections the brakes are objected to and must be replaced. An expensive solution to the problem is to equip the vehicles with ceramic or all-metal brakes made of coated aluminum or stainless steel, pushing up the price of the vehicle considerably.
Grey cast iron rotor corrosion affects not only the lifetime but also particle emissions. Corroded rotors cause a significant increase in particulate numbers and mass by 50% or more [3].
Consequently, brake rotors require not only a hard and thermally stable surface, but this surface should also have superior resistance to corrosion especially caused by salt water during wintertime.
Challenges of integrating new technologies into mass production
Nitrex’s unique NITREG®-C is a nitrocarburizing process based on the proven NITREG® potential-controlled gas nitriding technology. It incorporates the simultaneous diffusion of carbon and nitrogen into the steel surface.
NITREG®-C is often specified in industrial applications on the merit of it being an environmentally friendly but equivalent alternative to salt bath nitrocarburizing. The purpose of the treatment is to create a hardened superficial layer, enhancing wear and corrosion resistance, or improving fatigue resistance of treated steel or cast-iron parts, without distortion of shape or dimensional changes.
When resistance to corrosion and an attractive black finish are required, in-situ post-nitrocarburizing oxidation ONC® is the best technology.
ONC® is a clean technology producing a magnetite iron oxide layer that, in many instances, can replace chrome plating and salt bath nitriding with their inherent problems of pollution and cost. Depending on the type of steel, parts treated with the NITREG®-C + ONC® processes can easily pass well over 200 hours of salt spray test per ASTM B117 before the first corrosion spot appears.
Having been active in heat treating for decades and originally specializing in nitriding and nitrocarburizing, Nitrex does have a huge amount of experience in both fields, building the equipment but also operating it in the company’s commercial heat-treating facilities around the world. Based on this experience and constant research focusing on mechanical and corrosion properties, the findings of a research project that started in 2017 will be presented in the following text.
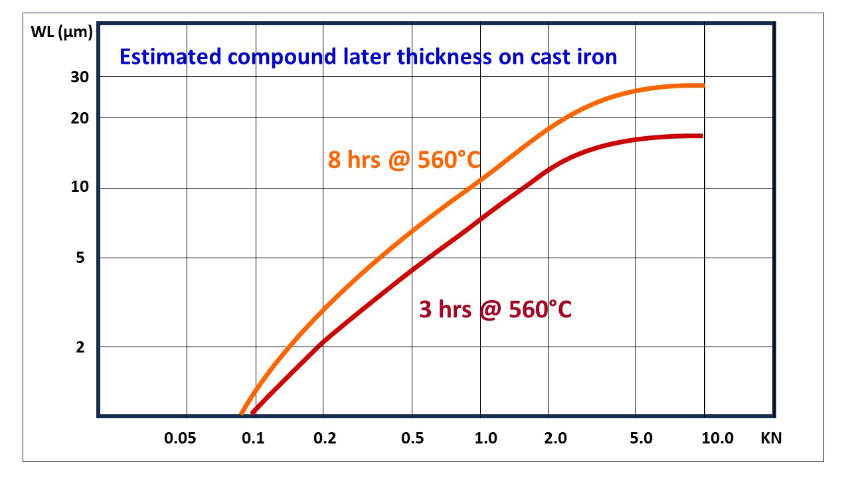
The extremely hard compound layer—compared to the base material—needs to be supported by the nitrogen-saturated diffusion layer giving a hardness profile, as shown in figure 2, to mitigate the risk of the so-called eggshell effect on soft substrates.
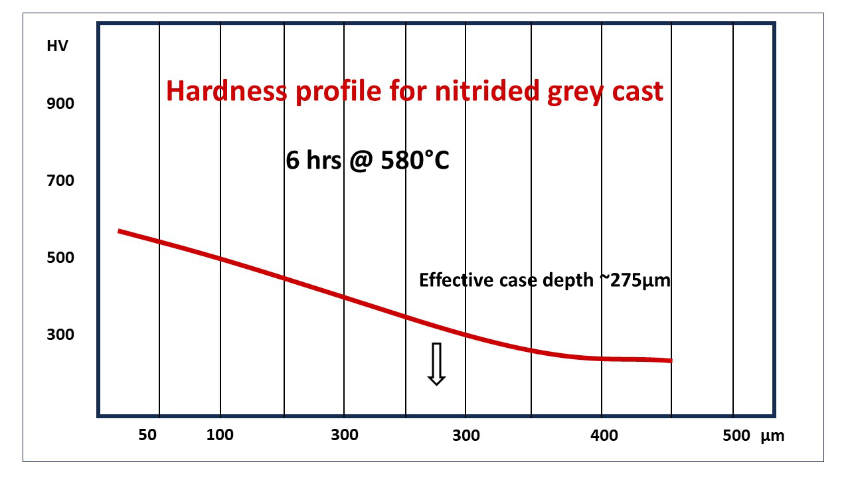
An additional requirement to produce highly reliable nitrocarburized cast iron components is a fast cooling from process temperature to minimize iron nitride precipitation. Iron nitrides are very brittle and promote flaking of the hard surface layer.
To present how Nitrex technology changes the mechanical properties of grey cast iron (GCI) brake rotors, four samples cut from rotors have been processed as shown on figure 3. Figure 4 presents the microstructures, showcasing the FNC layers of the samples.
Figure 3. Rotor samples used for evaluation with 20 μm (0.00079”) compound layer thickness
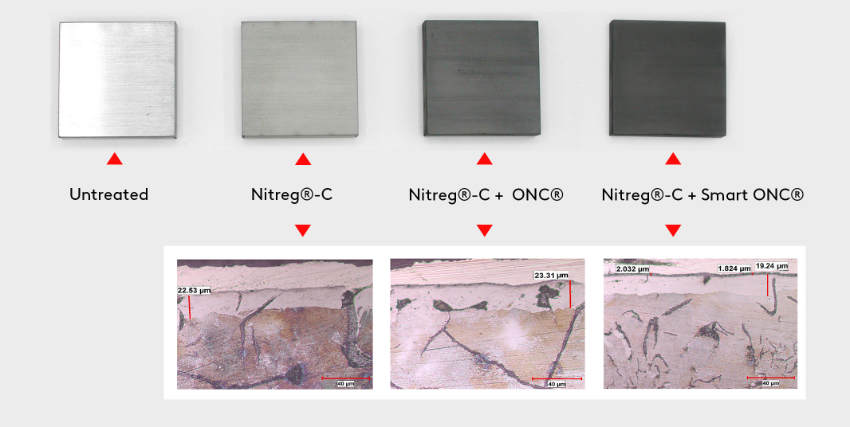
Figure 4. Compound layer of FNC samples
Corrosion tests in a salt spray chamber according to ASTM B117 (figure 5) displayed that untreated rotor material (left) as well as FNC-only treated rotor material (right) already show heavy corrosion after 20 hours.

Figure 6 shows specimens after 100 hours of oxidation (left) and Smart oxidation (right) in a salt spray chamber. It can clearly be observed that smart oxidizing is providing the highest corrosion resistance of all samples.

Future outlook on brake wear particle emission reduction, including the role of automation
Over the last 10 years, Nitrex has performed an extensive research project into wear-resistant nitrocarburized, nitrocarburized, and post-oxidized surfaces on cast and steel parts. This optimized oxidation layer is toxic-free, and does not contain chromium, nickel, cadmium, lead, barium, or mercury. It can withstand up to 560 °C (1,040 °F) and still provides excellent corrosion resistance without crystallization or any decomposition during heat cycles.
While evaluating ASTM 117B test results on GCI rotors, 120 hours can pass before seeing a first corrosion spot on rotor material. In comparison, a traditionally post-oxidized grey cast iron additionally treated with a rust inhibitor, typically fails after 48 hours. Commercially available GCI rotors with rust inhibitor paint (premium black paint) and plain OE models showed less than 24 hours of salt spray corrosion resistance. As for a zinc and aluminum flake basecoat, it is claimed to take between 300 and 480 hours of saltwater exposure before the rotors are totally corroded; rotors can last a bit longer if a topcoat is applied. Applying an electrophoretic coating with resin lasts 120 to 300 hours and a titanium grey finish provides 120 hours of salt spray protection.
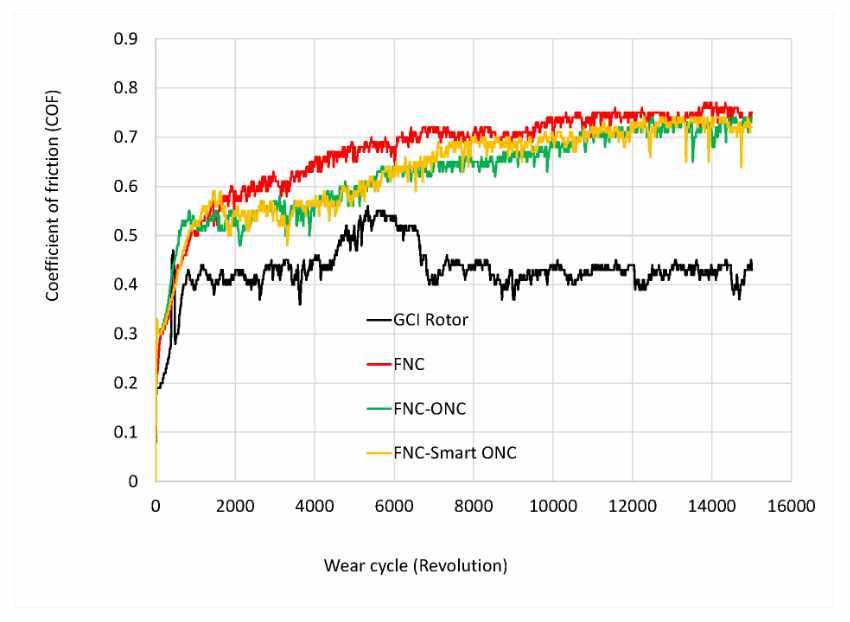
Pin-on-disc testing with 20 N (figure 7) shows that grey cast rotors treated with NITREG®-C have a higher coefficient of friction, stabilizing at 0.7, compared to untreated rotor material being somewhat stable at 0.42. All nitrocarburized samples show an almost identical behavior.
AUTOMATION
With nitrocarburizing being an additional production operation to standard brake rotors today, the constant rise of production costs driven by factors, like direct and indirect labor, as well as gas and energy prices, will add a non-negligible share to the total cost of braking systems. Keeping this in mind, alongside the sheer volume of parts to be treated, requires the installation of highly automated production centers as illustrated in a typical layout shown in figure 8.
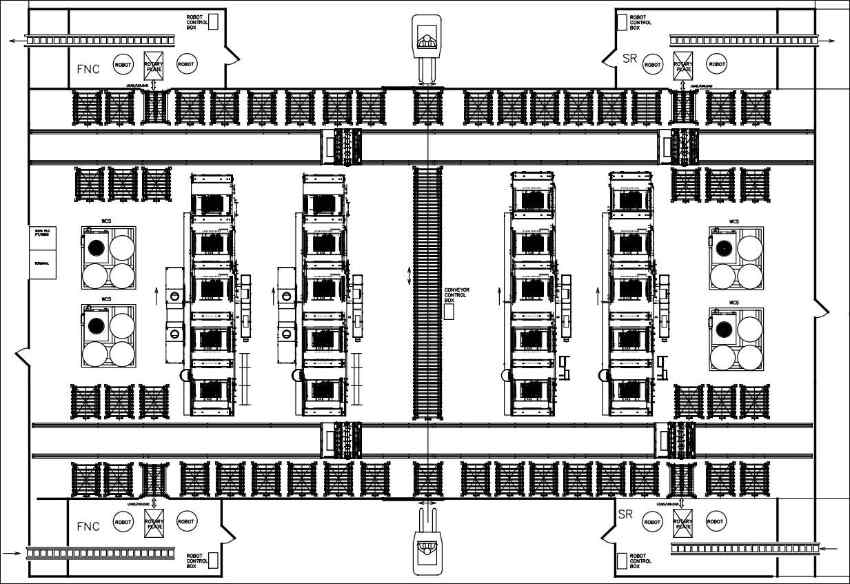
Besides the automation level to keep up with the high production level, this also requires a technology able to produce the brake rotor protection layers reliably time after time. Nitrex’s approach to high-volume mass production offers specifically designed multi-chamber furnaces, such as the NXL depicted in figure 9, where loads up to 3 metric tons are moved through sealed chambers, each of them performing a different stage of the FNC process. Starting with pre-heating, activating and nucleation, the load will then move to a first FNC chamber, continue to a second FNC chamber before being transferred to optional ONC® and fast cooling.

The cell is designed to operate so-called dry machined parts, which lets you skip the cleaning operation prior to nitrocarburizing (FNC) and/or stress relieve (SR). It is divided in two sections, FNC and SR, and It accommodates 50 racks, enabling lights-out operation for up to 30 racks during non-working hours. This setup ensures enhanced productivity and maintains overall production continuity, even in the event of an incident as described above. Parts are introduced into the cell from below, emerge from the top of the schema, and are conveyed in and out of the cell via roller conveyors.
The system employs redundancy concepts for incident handling, ensuring production continuity through strategic equipment placement and operational backup. Entry and exit points, along with SR and FNC lines, feature dual robots and furnaces to take over tasks in case of a malfunction, supported by a transverse line and manual ports for alternative loading and unloading. This design allows for a continuous production rate, even during operator-less shifts, by holding and automatically processing a set number of racks.
Missed the First Installment?
‘UNDERSTANDING BRAKE WEAR PARTICLE EMISSIONS AND EURO VII STANDARDS’ addresses the impact of brake emissions on urban particulate matter and pollution, with a focus on the European Commission’s Euro VII standards aimed at regulating these emissions. It explores the concerns over non-exhaust sources such as brakes and introduces initial technological solutions for mitigation.
https://fineeng.eu/part-1-understanding-brake-wear-particle-emissions-and-euro-vii-standards/
REFERENCES
- Aluminium-Bremsscheiben für elektrifizierte Personenkraftwagen? – Cuvillier Verlag.
- Rear brake disc scoring and corrosion – Volkswagen Forum.
- Ghouri, I., “The effects of corrosion on particle emissions from a grey cast iron brake disc”, presentation at EuroBrake 2022.

